Why does it MATTER? A WebQuest for 5th Graders |
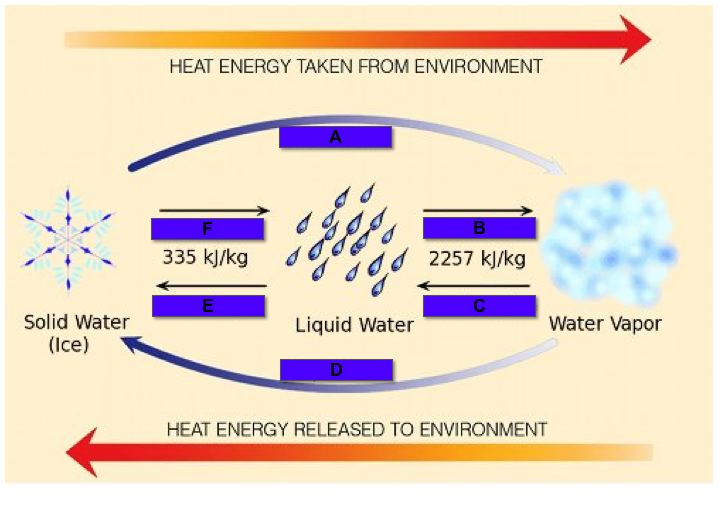
All matter can move from one state to another. It may require very low temperatures or very high pressures, but it can be done. Phase changes happen when certain points are reached. Sometimes a liquid wants to become a solid. Scientists use something called a freezing point to measure when that liquid turns into a solid. There are physical effects that can change the freezing point. Pressure is one of those effects. When the pressure surrounding a substance goes up, the freezing point also goes up. That means it's easier to freeze the substance at higher pressures. When it gets colder, most solids shrink in size. There are a few which expand but most shrink.
|